
GenesisCare Research provides patients access to clinical trials investigating emerging therapies and technologies in the management and treatment of cancer.
Participation is made possible through GenesisCare’s more than 45 clinical sites around Australia, which provide care in the clinical areas of Radiation Oncology, Theranostics, Medical Oncology, Haematology and Precision Medicine.
GenesisCare’s clinical research program provides end-to-end research services to support and facilitate research for industry sponsored, collaborative and investigator-initiated trials, in addition to real world evidence projects. We employ more than 55 dedicated research staff and work with over 150 Principal Investigators.
Our clinical trial start up timelines and accrual metrics are amongst the fastest and highest in the country, reflecting our commitment to excellence and efficiency in clinical research. We are often one of the first sites globally or nationally to be site activated including enrolling the first patient.
Centralised feasibility process where all feasibilities are managed by a dedicated team who liaise with the applicable sites and investigators on feasibility completion.
Feasibilities can be reviewed on a site/investigator basis or across multiple sites/investigators. See below for detailed information on site locations and capabilities.
To submit a feasibility questionnaire, please email research.feasibilities@genesiscare.com.
Centralised REG process including one contract, one budget, one indemnity, one ethics approval, one consent and one governance authorisation for each study being conducted across multiple sites. Service Agreements are in place with all external vendors ensuring fast budgeting, contracting and start up timelines.
Our clinical trial start up timelines and accrual metrics are amongst the fastest and highest in the country, reflecting our commitment to excellence and efficiency in clinical research.
Our study and site metrics - Coming Soon
Download the GenesisCare Research Information Pack for more information.
GenesisCare sites are fully equipped to conduct all phases of clinical research.
We have:
- 24 hour secure and restricted access areas
- Biological specimen collection, processing and storage
- Investigational Product dispensing and storage
- Refrigerated centrifuges, fridges, and freezers (-20 and -80 degrees with UPS backup power at main sites)
- Temperature controlled and alarmed cupboards, fridges, and freezers with remote notifications and centralised, web-based real-time monitoring and reporting
- Annual calibration and servicing of site equipment
- ECG machines and vital signs equipment
- Access to specialist nuclear medicine and radiopharmacy services (at applicable sites)
- Office facilities for sponsor representatives including access to phone, fax, photocopier, computer and wi-fi
- Centralised electronic medical record platform used across all GenesisCare clinical sites
- CTMS and SiteDocs eISF across all sites
- Multiple external vendors with multi-year service agreements.
We have partnerships with a number of public, private and academic organisations across Australia to help bridge the access gap. These partnerships include Public Private Partnerships with regional and metropolitan hospitals where we provide radiation services; Joint Ventures with imaging and medical service providers; partnerships with academic organisations for grant and ongoing professional development opportunities; and partnerships with external clinicians/researchers from hospitals and academic organisations who wish to collaborate with GenesisCare to undertake research.
If you are an external clinician/researcher wanting to work with GenesisCare, please find further details here.
To submit a research proposal, please click here
Our Quality team ensures that our trial operations are underpinned by Good Clinical Practice, the National Clinical Trial Governance Framework, local and international regulations and internal policies and procedures.
To assist with providing a high-quality service we have adopted various digital technology platforms such as CTMS and eConsent systems. Sponsors will be provided access to applicable systems to enable easy access to study and quality documentation.
We welcome external audits by sponsors, HRECs, and regulatory agencies as valuable opportunities for independent feedback, informing our growth and ongoing development. Our internal audit framework ensures we continuously monitor our systems and processes so that deficiencies are identified and addressed, supporting a high-quality clinical research service.
Please find the GenesisCare Research Statement of Endorsement for NCTGF: GC Statement of Endorsement for NCTGF_17Sep23.pdf
All staff undergo a rigorous onboarding process including a 90-day roadmap which addresses GenesisCare’s policies and procedures, GenesisCare Research SOPS and Work Instructions, industry short courses and an introduction to all systems utilised by GenesisCare.
There is also a comprehensive suite of competency documents with recognition of prior learning to ensure that staff meet the GenesisCare standards of practice. We work closely with EviQ, A-CTEC, PRAXIS and DataPharm to ensure that all staff are trained in line with industry standards. A buddy/mentor is provided for all new staff to assist with integration into the research team and into GenesisCare.
Regular newsletters are developed and distributed keeping the team informed of research highlights, business improvements, upcoming training and development opportunities, Policy/SOP revisions and required mandatory training.
All staff are GCP and IATA certified and undertake continuous professional development, both internal and external, ensuring high staff retention and the delivery of high quality care.

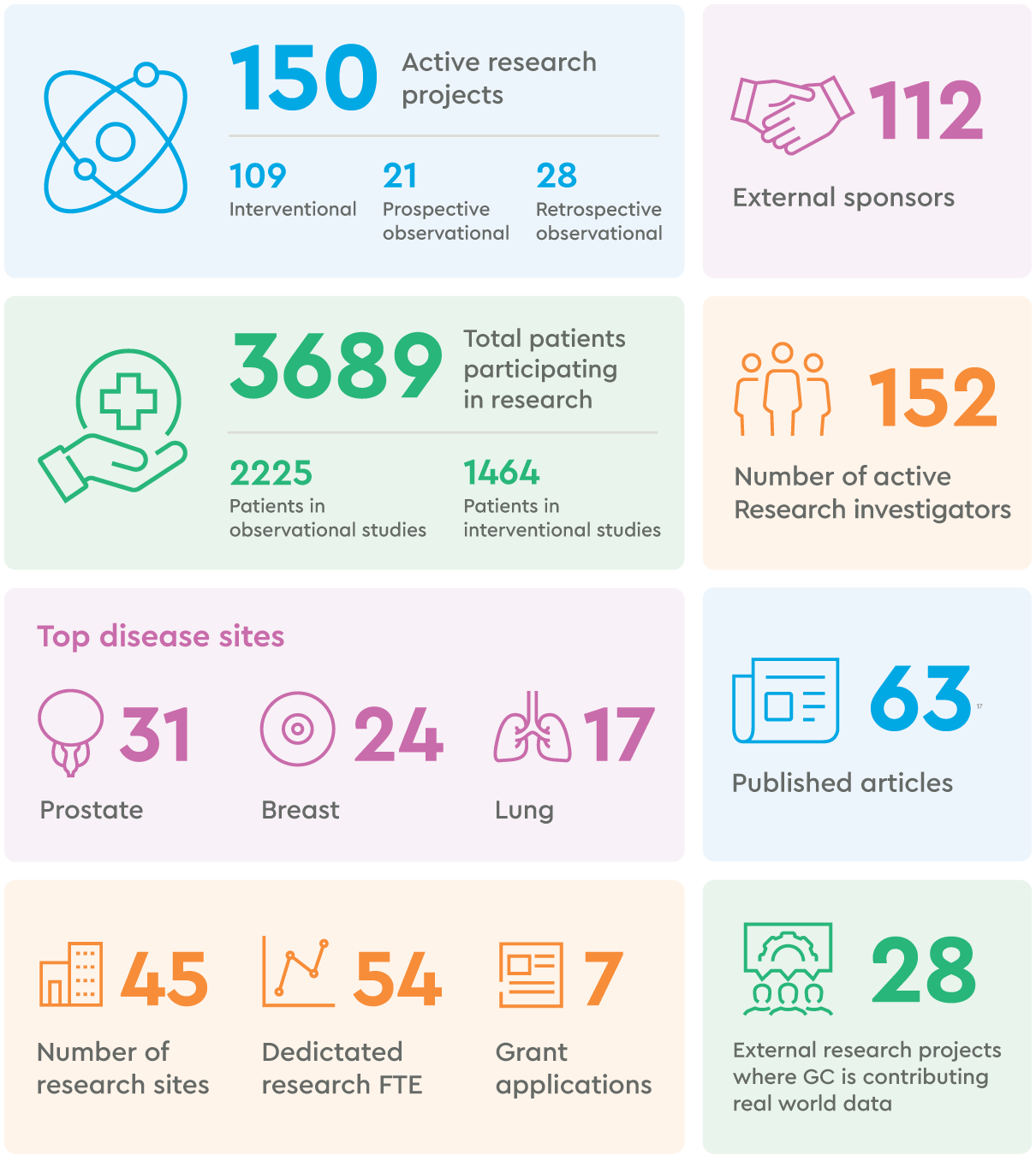
Research in numbers
Research highlights from 2024
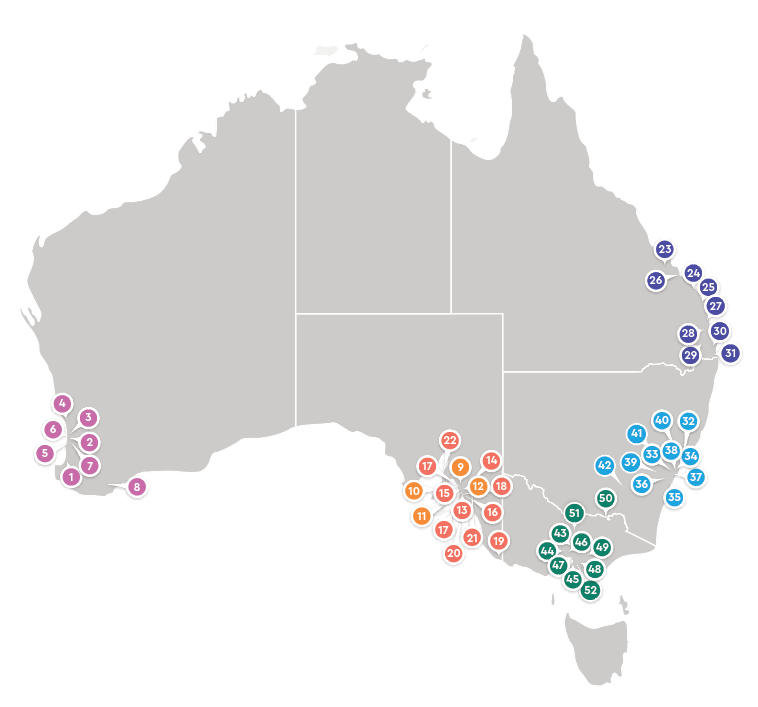
Locations & capabilities
We have a network of more than 50 locations across Australia.
Feasibility questionnaires
For feasibility questionnaires please submit to:
Research proposals
Meet our Clinical Research Leadership Team

Sonya McColl
Head of Research

Sonya McColl
Head of Research
Sonya brings over 30 years of expertise in healthcare, clinical research, and executive leadership. Beginning her career as a Registered Nurse in both Australia and the UK, she transitioned to clinical research in 2002. Among her many achievements, she transformed a small clinical trials unit with 3 staff and 7 projects into a state-of-the-art facility with 20 staff managing over 80 research projects.
In 2011, she founded McColl Clinical Research Consultancy, offering research services and advice to research sites, doctors and companies. Sonya has also played a pivotal role in establishing national research networks, first in cardiology and most recently in oncology, within GenesisCare. Sonya continues to work with GenesisCare today where she leads the national GenesisCare Research team of over 55 staff across 50 sites.

Head of Research
Head of Research
Sonya brings over 30 years of expertise in healthcare, clinical research, and executive leadership. Beginning her career as a Registered Nurse in both Australia and the UK, she transitioned to clinical research in 2002. Among her many achievements, she transformed a small clinical trials unit with 3 staff and 7 projects into a state-of-the-art facility with 20 staff managing over 80 research projects.
In 2011, she founded McColl Clinical Research Consultancy, offering research services and advice to research sites, doctors and companies. Sonya has also played a pivotal role in establishing national research networks, first in cardiology and most recently in oncology, within GenesisCare. Sonya continues to work with GenesisCare today where she leads the national GenesisCare Research team of over 55 staff across 50 sites.

Sharon Ray
Clinical Research Team Manager

Sharon Ray
Clinical Research Team Manager
Sharon joined Perth Radiation Oncology in 2004 (which was later acquired by GenesisCare in 2011) as a Registered Nurse. She went on to complete four years as a Research Nurse.
In 2013, Sharon became the Nurse Unit Manager for a new centre and remained in this role for five years before being promoted to the Director of Nursing for WA. Sharon maintained her links with research through her participation in national and state research committees before returning to the world of research in 2022 as the Clinical Research Team Manager.
Sharon is responsible for leading the site Clinical Research Coordinators and Assistants across the country to ensure the successful coordination of research projects.

Clinical Research Team Manager
Clinical Research Team Manager
Sharon joined Perth Radiation Oncology in 2004 (which was later acquired by GenesisCare in 2011) as a Registered Nurse. She went on to complete four years as a Research Nurse.
In 2013, Sharon became the Nurse Unit Manager for a new centre and remained in this role for five years before being promoted to the Director of Nursing for WA. Sharon maintained her links with research through her participation in national and state research committees before returning to the world of research in 2022 as the Clinical Research Team Manager.
Sharon is responsible for leading the site Clinical Research Coordinators and Assistants across the country to ensure the successful coordination of research projects.

Vicki Sproule
Research Operations Manager

Vicki Sproule
Research Operations Manager
Vicki Sproule joined GenesisCare in 2016 as the Research Manager for NSW and worked across several roles before becoming the Research Operations Manager in 2021. Vicki is a Registered Nurse and has worked in Oncology Research for the past 20 years.
In her current role Vicki is responsible for the management of all operational aspects of clinical research activities across the portfolios of Medical Oncology, Radiation Oncology, Haematology and Theranostics. Vicki has been able to develop a diverse range of skills that allow for the comprehensive understanding of the clinical research environment in Australia.

Research Operations Manager
Research Operations Manager
Vicki Sproule joined GenesisCare in 2016 as the Research Manager for NSW and worked across several roles before becoming the Research Operations Manager in 2021. Vicki is a Registered Nurse and has worked in Oncology Research for the past 20 years.
In her current role Vicki is responsible for the management of all operational aspects of clinical research activities across the portfolios of Medical Oncology, Radiation Oncology, Haematology and Theranostics. Vicki has been able to develop a diverse range of skills that allow for the comprehensive understanding of the clinical research environment in Australia.

Sarah Amos
Research Business Operations and Quality Manager

Sarah Amos
Research Business Operations and Quality Manager
Sarah Amos joined GenesisCare in 2021 as the Research Business Operations and Start-Up Manager and has since transitioned to the role of Research Business Operations and Quality Manager. With her foundation as a Radiation Therapist, Sarah has cultivated a robust expertise in oncology research and regulatory affairs. She has held senior management roles across both public and private sectors and earned a Master of Public Health, specialising in Health Economics and Financial Management.
In her current role, Sarah is responsible for overseeing research business operations, which includes key metric reporting, financial management, marketing, and the integration of new technologies. She is dedicated to ensuring that quality is a fundamental aspect of GenesisCare’s research program, enhancing the overall effectiveness and integrity of in house initiatives.

Research Business Operations and Quality Manager
Research Business Operations and Quality Manager
Sarah Amos joined GenesisCare in 2021 as the Research Business Operations and Start-Up Manager and has since transitioned to the role of Research Business Operations and Quality Manager. With her foundation as a Radiation Therapist, Sarah has cultivated a robust expertise in oncology research and regulatory affairs. She has held senior management roles across both public and private sectors and earned a Master of Public Health, specialising in Health Economics and Financial Management.
In her current role, Sarah is responsible for overseeing research business operations, which includes key metric reporting, financial management, marketing, and the integration of new technologies. She is dedicated to ensuring that quality is a fundamental aspect of GenesisCare’s research program, enhancing the overall effectiveness and integrity of in house initiatives.

Kathryn Hogan
Research Development Manager

Kathryn Hogan
Research Development Manager
Kathryn joined GenesisCare in 2021 as a Research Lead. She quickly began to focus on investigator-initiated research and was promoted to Research & Development Lead - IIT and then Research Development Manager.
Kathryn's background in science includes completion of Honours working on Mesothelioma in the laboratory. She then started her first role as a Clinical Research Coordinator in a busy medical oncology trial unit at a private hospital. Realising her passion for clinical research, Kathryn went on to complete a Master of Clinical Trials Research which led to work at a medical research institute prior to joining GenesisCare. Kathryn's aim is to facilitate innovative research ideas within the organisation and foster research collaborations that lead to better outcomes for patients at GenesisCare.

Research Development Manager
Research Development Manager
Kathryn joined GenesisCare in 2021 as a Research Lead. She quickly began to focus on investigator-initiated research and was promoted to Research & Development Lead - IIT and then Research Development Manager.
Kathryn's background in science includes completion of Honours working on Mesothelioma in the laboratory. She then started her first role as a Clinical Research Coordinator in a busy medical oncology trial unit at a private hospital. Realising her passion for clinical research, Kathryn went on to complete a Master of Clinical Trials Research which led to work at a medical research institute prior to joining GenesisCare. Kathryn's aim is to facilitate innovative research ideas within the organisation and foster research collaborations that lead to better outcomes for patients at GenesisCare.

Dr Julie Rowe
Research Ethics and Governance Manager

Dr Julie Rowe
Research Ethics and Governance Manager
Julie joined GenesisCare in 2022 as a Senior Research Ethics and Governance Specialist and became the Research Ethics and Governance Manager in 2023. Julie has a background in science, having completed a PhD in immunology and an 11-year lab-based postdoctoral appointment. Julie then made the move to oncology clinical trials, working initially as a Clinical Trial Coordinator and then the Clinical Trials Manager within both public and private hospitals. During this time she also helped to establish a new Oncology Clinical Trials Unit.
Julie worked for 2 years as a coordinator for a private Human Research Ethics Committee (HREC) and has experience in the operational aspects of a HREC. With her combined experience, Julie’s aim is to help facilitate the timely start up activities associated with research in order to improve the access of patients to cutting edge research and treatments.

Research Ethics and Governance Manager
Research Ethics and Governance Manager
Julie joined GenesisCare in 2022 as a Senior Research Ethics and Governance Specialist and became the Research Ethics and Governance Manager in 2023. Julie has a background in science, having completed a PhD in immunology and an 11-year lab-based postdoctoral appointment. Julie then made the move to oncology clinical trials, working initially as a Clinical Trial Coordinator and then the Clinical Trials Manager within both public and private hospitals. During this time she also helped to establish a new Oncology Clinical Trials Unit.
Julie worked for 2 years as a coordinator for a private Human Research Ethics Committee (HREC) and has experience in the operational aspects of a HREC. With her combined experience, Julie’s aim is to help facilitate the timely start up activities associated with research in order to improve the access of patients to cutting edge research and treatments.
