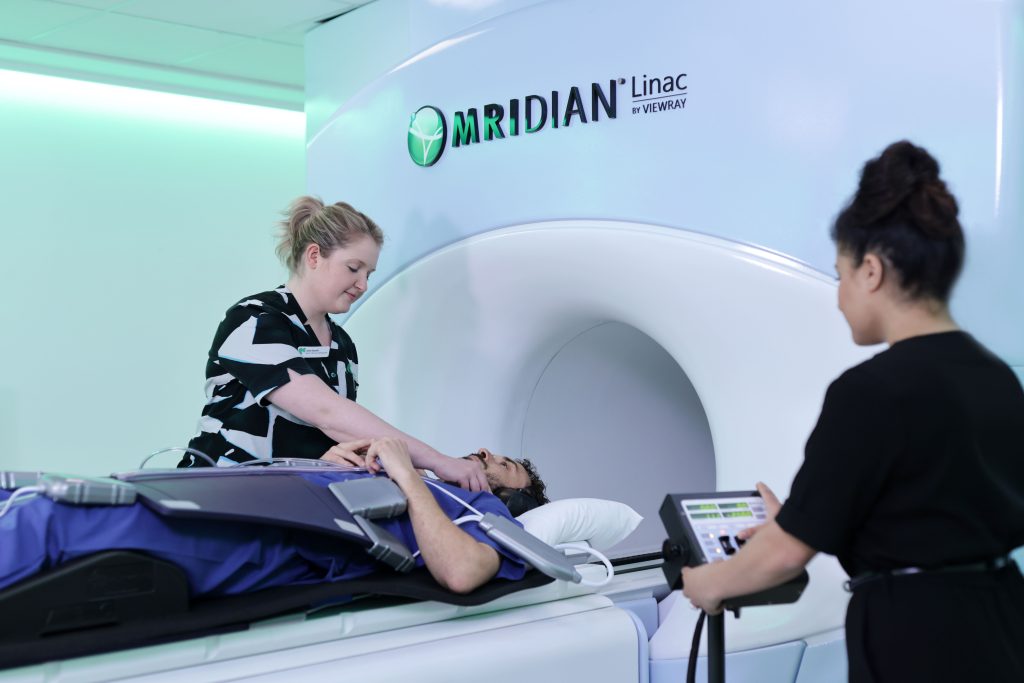
Annual research report 2020
GenesisCare is delighted to launch our 2020 Annual Research Report, which provides a snapshot of key research highlights and clinical trials activity from across the GenesisCare network. In light of our growing global research footprint, this year we have expanded our Annual Research Report to include clinical research activity in all the countries in which we operate – Australia, the United States, the United Kingdom and Spain.
2020 was a year like no other. COVID-19 temporarily shut down the world and placed significant pressure on healthcare systems and the research sector.
Our research teams found new and improved ways to ensure the continuity of potentially life-saving clinical trials, rapidly adapting to the ever-changing COVID-19 landscape.
Despite the challenges from the pandemic, we recruited more than 700 new patients into our trials across cardiology and oncology in 2020. In total, 2,258 patients were part of our follow up trials across the globe.
A huge thank you must go out to our research partners who have played an important role in ensuring the continuity of critical research and we thank you for your ongoing support and collaboration.
Read our GenesisCare Annual research report for Oncology research in the UK
GenesisCare Global Footprint

GenesisCare Research Highlights
In this report we will take a look back on some of our key research achievements from 2020.
Our team in Spain partnered with La Milagrosa Hospital in Madrid to design a prospective study of Ultra-Low Doses of Therapy with Radiation Applied to COVID-19 (ULTRA-COVID) for patients who suffer pneumonia who are not candidates for ventilation.
In a truly global research effort, GenesisCare was the highest recruiter for Barrigel, Palette Life Sciences’ FDA clinical trial, enrolling patients at GenesisCare centres across Australia, the USA and Europe. Our Australian team worked closely with Spanish investigators in relation to the study set up, recruitment and training – a great example of the value of our global reach in clinical research.
In Oncology Australia, GenesisCare was the highest enrolling site globally (Fiona Stanley Hospital) for the DASL Hi-CaP study, which is assessing whether a novel hormone treatment added to standard therapy can lessen the risk of recurrence of prostate cancer.
GenesisCare also received approval to expand the DEPART trial internationally to the Netherlands, which is a GenesisCare sponsored study assessing radiotherapy for the management of Dupuytren’s disease.
Our research function at GenesisCare was born 20 years ago in our cardiology department in Australia. It has now grown to over 32 clinical investigators, 25 dedicated research staff, and nine cardiology research sites. In 2020, GenesisCare continued to be a partner of choice and leading global recruiter on several global cardiology trials, including the OPTIMSER, SURPASS and CATALYST clinical trials.
Looking ahead
Looking ahead, the future of research at GenesisCare is an exciting one. In 2021 we welcomed Dr Wally Curran, MD, as our Global Chief Medical Officer, a highly respected and world renowned expert and researcher in oncology. Dr Curran has held leadership positions at several leading academic institutions in the United States, including NRG Oncology, a National Cancer Institute – a funded national cancer clinical trials cooperative group.
Under Wally’s leadership, we recently launched our new Research & Insights Business Division which will allow us to provide a truly integrated research model, combining real world evidence, our contract research organisation and site activities across the globe.
Our newly established Research & Insights Business Division will have a strong focus on expanding our research portfolio in the United States and across the globe, partnering with leading institutions such as NRG Oncology on multi-national clinical trials.
We look forward to working with all of our research partners and clinical trial participants in 2021 to deliver the best possible life outcomes for the patients of today and tomorrow.