MR linac for challenging SABR cases
The use of stereotactic ablative radiotherapy has increased in recent years and supportive data from randomised studies have recently been published.
The SABR-COMET phase II multicentre trial investigated patients with oligometastatic disease from prostate, breast, lung and colorectum. It showed an increase in survival in patients with between 1 and 5 metastases. Median overall survival was 41 months for patients treated with SABR compared to 28 months in the standard treatment arm. Progression-free survival was 12 months in the SABR arm compared to 6 months in the standard radiotherapy arm.
The use of MRIdian to deliver SABR to challenging anatomical locations particularly in the re-treatment setting, is made easier thanks to the rapid online adaptation of OARs and target and plan reoptimisation.
My patient, Mr B was diagnosed with prostate cancer in 2010 when his PSA was found elevated at 6.5. He underwent radical prostatectomy with final histology showing adenocarcinoma, Gleason 3+4 with extracapsular extension, pT3a and N pelvic nodes positive but surgical margins clear.
He had biochemical recurrence a year later and was treated with salvage prostate bed radiotherapy at the conventional dose of 66Gy in 33 fractions.
The PSA responded well and nadired at <0.1 but in May 2020 increased to 0.23 and repeated in August, to 0.3.
The PSMA PET performed in August showed two avid pelvic nodes compatible with recurrent disease (Figure 1 and 2).
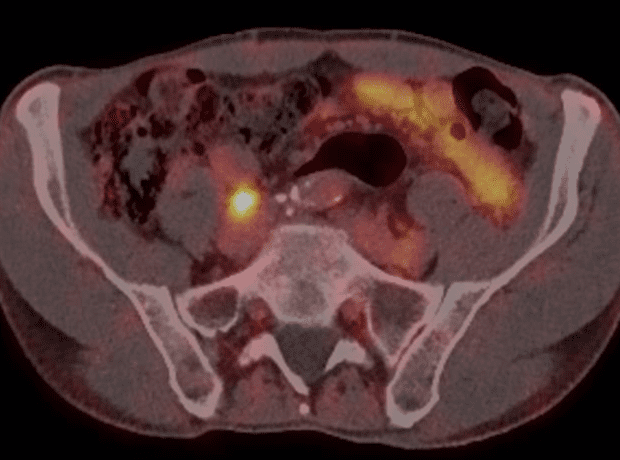
Figure 1. Right common iliac node
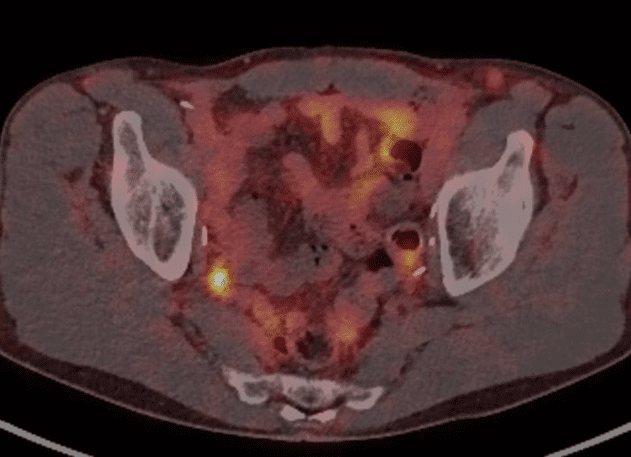
Figure 2. Right internal iliac node
The lower internal iliac node was close to the previous radiotherapy fields (Figure 3). This challenging location made the delivery of SABR difficult due to the risk of damaging the adjacent small bowel. For this reason, with the support of the SABR Advisory Team MDT, it was decided to treat the patient with MRIdian to achieve a high dose coverage to both of the lymph nodes whilst maximally sparing the bowel.
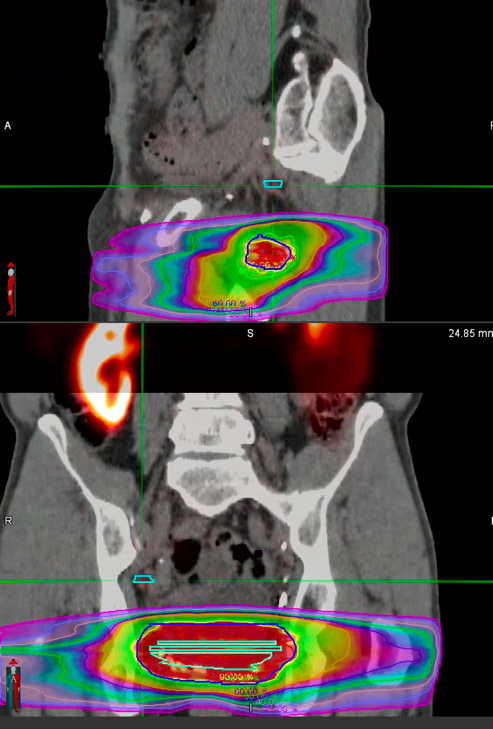
Figure 3. Previous RT fields, nodal disease outlined in blue.
Mr B was planned to receive 30Gy in 5 fractions to the lower node (Figure 4) and 40Gy in 5 fractions to the higher one (Figure 5), on alternate days. The plan achieved all the mandatory OAR constraints.
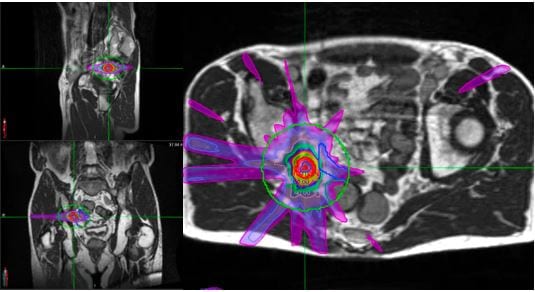
Figure 4. Lower node MRL plan with bowel constraints met
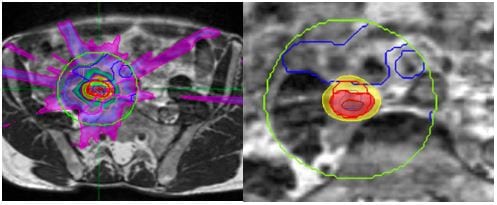
Figure 5. Higher node MRL plan with bowel sparing
Mr B was planned to receive 30Gy in 5 fractions to the lower node (Figure 4) and 40Gy in 5 fractions to the higher one (Figure 5), on alternate days. The plan achieved all the mandatory OAR constraints.
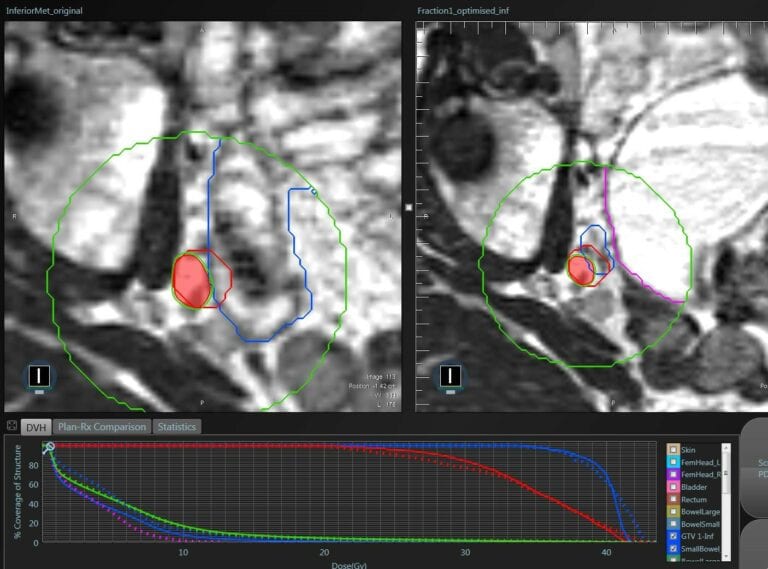
Figure 6.Daily re-optimisation according with bowel movements
The treatment was safely delivered, and Mr B did not experience any acute side effects during treatment. He will repeat his first PSA at 6 weeks and will be monitor with PSA readings and toxicity check via PROMs and clinical review every 3 months.
In conclusion, SABR using MRIdian is a safe and efficient modality for the treatment of selected oligometastatic patients. MRIdian offers a superior soft tissue definition in comparison to the CBCT and a rapid online adaptation that is required in challenging cases especially in the re-irradiation setting.
Click here for more information on SABR
Dr Carla Perna
Clinical Oncologist and MRIdian specialist
MBBS, FRCR
Special clinical interest in Prostate Cancer, Brachytherapy & Testicular cancer


