Simultaneous ablation of five liver mets
A MRIdian case study
Oligoprogressive disease (OPD) is a distinctive form of oligometastatic disease. (1) OPD describes progression at only a few sites with or without new sites of metastases, with otherwise controlled primary and distant disease, following an initial response to treatment. This is commonly seen in patients undergoing molecular targeted therapy but can occur with any form of systemic anti-cancer therapy (SACT). Local ablative therapy for OPD may delay the development of wide-spread resistant disease and allow the continuation of their existing systemic treatment by destroying the resistant sub-clones. There is evidence from retrospective studies (2,3) and single centre experience (4) to support the idea that local ablative therapy can safely and durably eradicate isolated sites of progression, leading to improved progression free survival (PFS) and hopefully overall survival (OS). However, randomised trial data to support this hypothesis is currently limited (5,6,7) but will be enriched when future clinical trials report (HALT, TRAP).
Stereotactic ablative radiotherapy (SABR) allows us to ablate targets smaller than 5 – 6cm with high radiation doses. Irradiation of multiple liver metastases with SABR aims to provide local control, and delay the need for changing existing systemic therapy in the short term, while sparing the adjacent organs at risk (OARs) and thus reducing unwanted side effects.
The ViewRay MRIdian is a state-of-the-art SABR platform that combines an MR scanner with a linear accelerator. It provides superior soft tissue resolution and allows for on-table adaptation of radiotherapy plans to account for the daily change in position of a tumour and nearby organs at risk. In addition, the use of real-time gated treatment delivery in breath-hold can result in improved tumour control and reduced toxicity.
My patient Mr AS presented in 2019 with a primary cancer of the caecum, liver, perihepatic nodal and possible lung metastases. Although liver metastases were resectable at presentation, it was not offered due to the presence of perihepatic nodal disease. Therefore he went ahead with a right hemicolectomy for his locally advanced primary caecal cancer. He started FOLFIRI plus Bevacizumab in July 2019 and tolerated chemotherapy very well and has managed to enjoy a good quality of life over the intervening 2-years. In September 2021 he remained well but unfortunately his restaging CT scan showed a total of 5 liver lesions. Although 3 were existing metastases, 2 of the metastases were new, confirming OPD (figure 1). The CT did not reveal any sites of progression elsewhere. Following discussion at the GenesisCare SABR MDT I had offered him MR-guided SABR to all of the liver metastases. He was also keen to be able to continue on his current SACT.
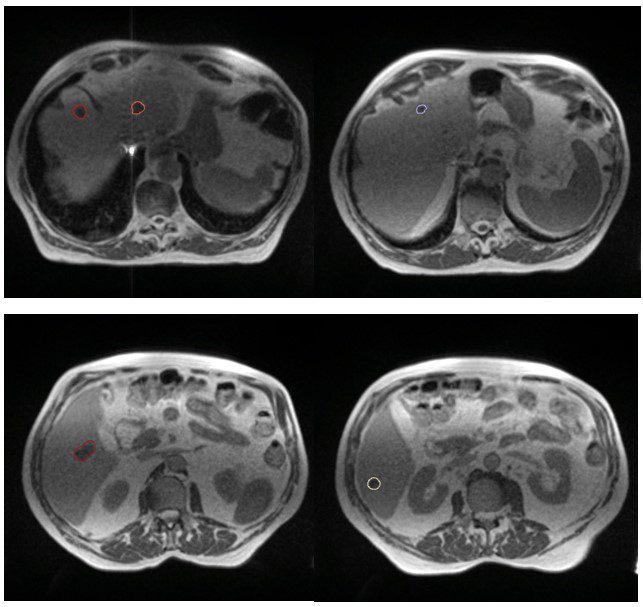
Figure 1: MR images of liver metastases
The SABR referral process at GenesisCare is swift and collegial and the high level of clinical governance in place is reassuring, given the complexity of this form of treatment. The advantages of the MRIdian platform in allowing daily GTV and OAR recontouring, plan reoptimisation and respiratory gated treatment delivery maximised target coverage and minimised dose to normal liver. At simulation the GenesisCare physics team noted that all the lesions were moving synchronously and all 5 lesions were therefore planned to be treated simultaneously in one plan. GTV was defined using a contrast enhanced diagnostic MRI. A CTV was created from his GTV by adding a uniform 3mm margin, which was then adjusted to obey the liver contour. The final PTV was created by adding an isotropic 5mm margin to the CTV except for CTV3 where a 3mm margin was used as this was going to be used as the index lesion for MRL treatment tracking and therefore did not warrant a 5mm margin.
He was treated with 45Gy in 5 fractions delivered on alternate days. The plan achieved all mandatory constraints including mean liver dose of less than 15Gy. Treatment was planned (figure 2) and delivered, with daily adaptation, consistently achieving coverage as planned, while respecting the dose constraints of the OARs (figure 3). During treatment delivery, any movement of the index PTV3 outside of a tightly defined ‘treatment reticule’ resulted in a pause in treatment until target re-entered the reticule. This gave us confidence that the entire planned dose was delivered to the targets. Overall the process was straightforward and the patient was supported throughout by the on-set MRLinac team. Apart from Grade 2 fatigue, he has not experienced any further toxicity. He was reviewed at 2 weeks post treatment and will have a response assessment scan in 8 weeks.
He was also GenesisCare’s 400th MRIdian patient and was very pleased with the care and compassion provided by the whole GenesisCare Cromwell team. Mr AS commented, “The treatment itself was painless, the main side effect being tiredness. GenesisCare has been fantastic and their care has gone beyond my treatment and extended to my direct family.”
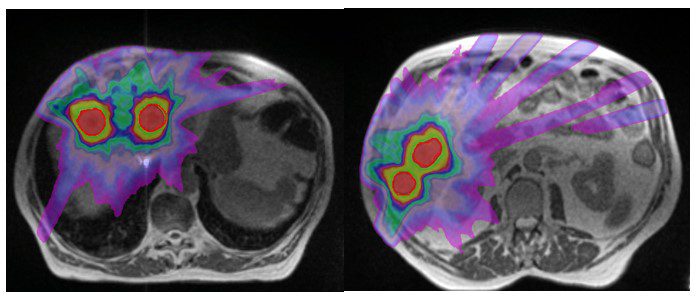
Figure 2: MR images of SABR plan with isodoses
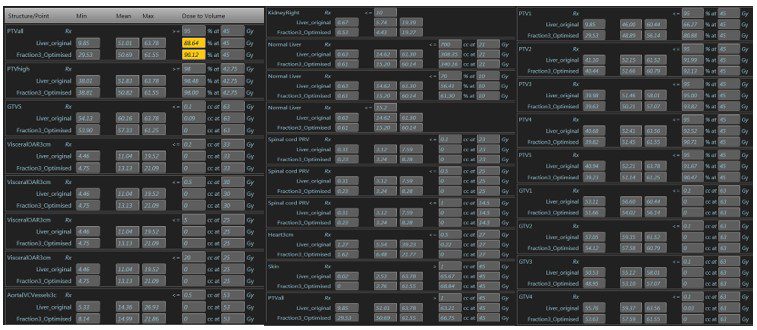
Figure 3: Base plan compared to adapted fraction no 3
This case report illustrates the flexibility of the MRIdian platform in delivering ablative radiotherapy to complex targets, putting the service at the forefront of modern cancer medicine in the UK.
If you would like more information about how to access these treatments for your patients, please don’t hesitate to contact sabr@genesiscare.co.uk
Kind regards,
Dr Rafiqul Islam
Clinical Oncologist and MRIdian Specialist
GenesisCare UK