Stereotactic radiotherapy for the management of early stage lung cancer
Stereotactic ablative radiotherapy (SABR) delivered on the MRIdian MR linac is a non-surgical treatment of choice for patients with centrally located early-stage lung cancer in our UK GenesisCare network. This article covers patient profiles, survival rates, the use of single fraction (1#) SABR and how SABR is being used for central/ultra central lung tumours.
Approximately 20% of non-small cell lung cancer (NSCLC) patients present with early-stage disease (T1N0 or T2N0), defined as tumours up to 5 cm in size, without nodal or distant metastases. Surgery has been considered the standard of care; however, many patients are ineligible because of co-morbid conditions such as chronic obstructive pulmonary or cardiovascular disease. Despite newer minimally invasive surgical techniques, 41% of patients are readmitted during the 90 days following surgery and there remains a 3% risk of death during this period¹. Consequently, some patients opt for non-surgical management.
SABR has been associated with high rates of local control, with 3-year local control of approximately 90% after SABR, comparable to results obtained with anatomic lobectomy³. Because of these promising outcomes, randomized trials are currently comparing SABR vs. surgery as first-line treatment for early-stage NSCLC. Currently the JoLT-Ca STABLE-MATES trial (NCT02468024) is recruiting patients considered as high risk for cardiothoracic surgery, with peripherally located stage I lung cancers and randomising them to sublobar resection or SABR (delivered in 3 fractions) in a phase III superiority trial with an endpoint of overall survival.
1# SABR
Extreme hypofractionation may be possible for tumours that are neither near central mediastinal structures nor adjacent to the chest wall. The long-term follow-up on NRG Oncology RTOG 0915 study of 34 Gy in a single fraction showed no difference in terms of local control or overall survival and lower rates of grade 3³ toxicity compared to a fractionated SABR schedule⁴. A systematic review of single fraction SABR highlighted the good rates of local control and low rates of toxicity⁵, and suggested that concern regarding the chest wall toxicity is unfounded. In fact, they even suggest the Royal College of Radiologists guidance of radiotherapy for NSCLC during the COVID-19 pandemic, of which I am a co-author, over emphasises the chest wall toxicity of single fraction SABR⁶. I am inclined to agree: chest wall pain following treatment of lesions abutting the chest wall is approximately 30% for fractionated or single fraction SABR and video-assisted thoracic surgery. No treatment is side effect free but the benefit of having a curative treatment for their lung cancer in a single treatment cannot be understated, especially for patients who have co-morbid conditions and where there is a long distance to travel for treatment. Interestingly this review discusses the limited uptake of single fraction SABR despite the evidence of its benefit and makes for a thought provoking read.
1# SABR can be delivered on both our conventional linac platforms and the MRIdian MR linacs at our Oxford and Cromwell centres. On a conventional linac platform the approach is to encompass the entirety of tumour motion during breathing using an internal target volume (ITV) with modern surface guidance to check for consistent breathing cycles and ensure consistent delivery of radiation to the tumour. The Amsterdam group have published how the ViewRay MRIdian platform can be used to deliver single fraction treatment⁷. MRIdian allows real-time adaptation of the target volume prior to treatment, and patients are treated in an inspiratory breath-hold, so an ITV approach is not needed, reducing the irradiated volume by 50%. In my experience watching patient involvement in the gating process is very gratifying. In the Dutch group’s case series of 10 patients treated with a single fraction of 34 Gy on the MRIdian, the treatment times ranged from 30-60 minutes which compares favourably with the treatment times of sublobar resection or therapeutic ablations.
MR-guided SABR for central and ultra-central lesions
Central and ultra-central tumours are also a key area of interest⁸. Definitions vary, but essentially:
- Central: Tumours located within 2 cm in all directions around the proximal bronchial tree and immediately adjacent to mediastinal or pericardial pleura with a PTV expected to touch or include the pleura.
- Ultra-central: Tumours where the planning target volume (PTV) touches or overlaps the central bronchial tree, oesophagus, or pulmonary artery
For these tumours, SABR is delivered in a more fractionated manner using 5-8 treatments. The main challenge is minimising the risk of toxicity for tumours whilst ensuring a therapeutic dose is delivered to ensure the high rates of local control (~90%) seen with SABR for more peripherally located tumour. The more tumour movement there is, the larger the area that needs to be irradiated which is where respiratory gating and treatment in breath hold using the MRLinac MRIdians really come into their own.
A recent patient of mine, referred by a cardiothoracic surgeon because of the high operative risk because of their co-morbid conditions, presented with a rapidly growing, ultra-central 3.5 cm squamous cell cancer, which had grown to 5.5cm by the time they started treatment. Because of the use of the MRIdian we were able to adapt the plan onset, deliver the treatment and avoid a delay for re-planning. The patient received 60 Gy in 8# on alternate days to minimise the potential of toxicity and to work around treatment for their other co-morbid conditions. Because the patient was treated on the MRIdian, daily adaptation to account for tumour growth during treatment was possible and treatment in breath hold meant that smaller margins (3mm vs 5mm) could be used. We were able to achieve OAR tolerances with minimal compromise on tumour coverage in a way that would not have been possible on a different platform. The patient remains in remission and nearly a year later the lesion has shrunk to 2cm and is no longer PET avid.
Figure 1: Cine loop of intrafraction imaging showing a small amount of tumour movement during respiration. Treatment in breath hold as indicated by ‘Beam On’. Intrafraction imaging allowed for small PTV margins (3mm versus 5mm) reducing overall treatment volume.
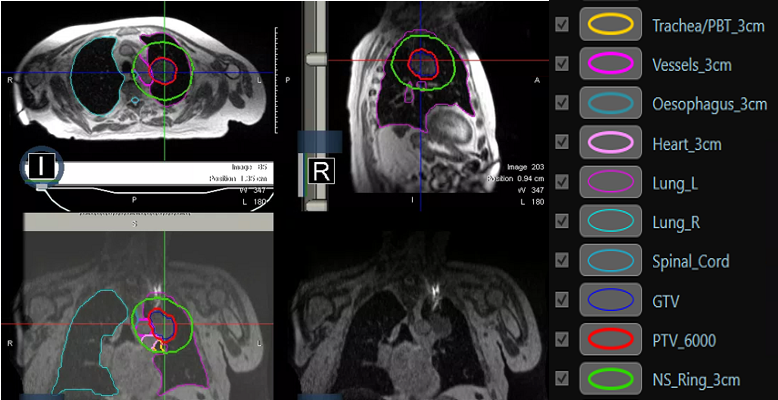
Figure 2: MRI planning and on treatment imaging prior to treatment. NS_Ring_3cm is the planning structure within which the GTV and any OARs are adapted daily by the on-set consultant of the day prior to each fraction.
The treatment of ultra-central lung cancers with SABR is an area of evolving clinical practice, whilst clinical trials to identify the appropriate dose of radiotherapy and number of fractions for treatment on conventional linear accelerators is ongoing (SUNSET TRIAL - NCT03306680), outcomes for treatment using daily adaptation on MRIdian are encouraging⁹. SABR using the MRIdian platform is the treatment of choice for the majority of patients with centrally located early-stage lung cancers treated in the GenesisCare network in the UK.
Refer today
To refer to one of our specialists or to find out how we can help you and your patients, contact us at the below email.
Dr Crispin Hiley
Clinical Director for Lung Cancer
GenesisCare UK
References
- Royal College of Physicians. National Lung Cancer Audit. Lung cancer clinical outcomes publication (for the 2018 audit period). London: Royal College of Physicians (July 2021) https://nlca.rcp.ac.uk/content/misc/LCCOP%202021(2018).pdf [Accessed February 2023]
- Phillips I, Sandhu S, Lüchtenborg M, Harden S. Stereotactic Ablative Body Radiotherapy Versus Radical Radiotherapy: Comparing Real-World Outcomes in Stage I Lung Cancer. Clin Oncol (R Coll Radiol). 2019 Oct;31(10):681-687.
- Chang JY, Senan S, Paul MA, et al: Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 16:630-7, 2015
- Videtic GM, Paulus R, Singh AK, Chang JY, Parker W, Olivier KR, Timmerman RD, Komaki RR, Urbanic JJ, Stephans KL, Yom SS, Robinson CG, Belani CP, Iyengar P, Ajlouni MI, Gopaul DD, Gomez Suescun JB, McGarry RC, Choy H, Bradley JD. Long-term Follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2019 Apr 1;103(5):1077-1084. doi: 10.1016/j.ijrobp.2018.11.051. Epub 2018 Dec 1. PMID: 30513377; PMCID: PMC6454873.
- Bartl AJ, Mahoney M, Hennon MW, Yendamuri S, Videtic GMM, Stephans KL, Siva S, Farrugia MK, Ma SJ, Singh AK. Systematic Review of Single-Fraction Stereotactic Body Radiation Therapy for Early Stage Non-Small-Cell Lung Cancer and Lung Oligometastases: How to Stop Worrying and Love One and Done. Cancers (Basel). 2022 Feb 3;14(3):790. doi: 10.3390/cancers14030790. PMID: 35159057; PMCID: PMC8834253.
- Faivre-Finn C, Fenwick JD, Franks KN, Harrow S, Hatton MQF, Hiley C, McAleese JJ, McDonald F, O'Hare J, Peedell C, Pope T, Powell C, Rulach R, Toy E. Reduced Fractionation in Lung Cancer Patients Treated with Curative-intent Radiotherapy during the COVID-19 Pandemic. Clin Oncol (R Coll Radiol). 2020 Aug;32(8):481-489. doi: 10.1016/j.clon.2020.05.001. Epub 2020 May 13. PMID: 32405158; PMCID: PMC7218369.
- Finazzi T, van Sörnsen de Koste JR, Palacios MA, Spoelstra FOB, Slotman BJ, Haasbeek CJA, Senan S. Delivery of magnetic resonance-guided single-fraction stereotactic lung radiotherapy. Phys Imaging Radiat Oncol. 2020 May 20;14:17-23. doi: 10.1016/j.phro.2020.05.002. PMID: 33458309; PMCID: PMC7807654.
- Chang JY, Bezjak A, Mornex F; IASLC Advanced Radiation Technology Committee. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol. 2015 Apr;10(4):577-85. doi: 10.1097/JTO.0000000000000453. PMID: 25514807.
- Regnery S, Buchele C, Weykamp F, Pohl M, Hoegen P, Eichkorn T, Held T, Ristau J, Rippke C, König L, Thomas M, Winter H, Adeberg S, Debus J, Klüter S, Hörner-Rieber J. Adaptive MR-Guided Stereotactic Radiotherapy is Beneficial for Ablative Treatment of Lung Tumors in High-Risk Locations. Front Oncol. 2022 Jan 11;11:757031. doi: 10.3389/fonc.2021.757031. PMID: 35087746; PMCID: PMC8789303.
